Prime Scientific - Concentration Determination by Means of Density Measurement
If there is a relation between the concentration of a substance in a solution and the density of the solution, the concentration can be determined by measuring the density of the solution.
The concentration of solution is directly related to its density. As concentration increases, density increases proportionally. More specifically, as the concentration of a solution increases, solute molecules become increasingly abundant. They then become dissolved in the solvent. Thus, the solution gains more mass per unit volume; the density and concentration also increases with an increase in mass.
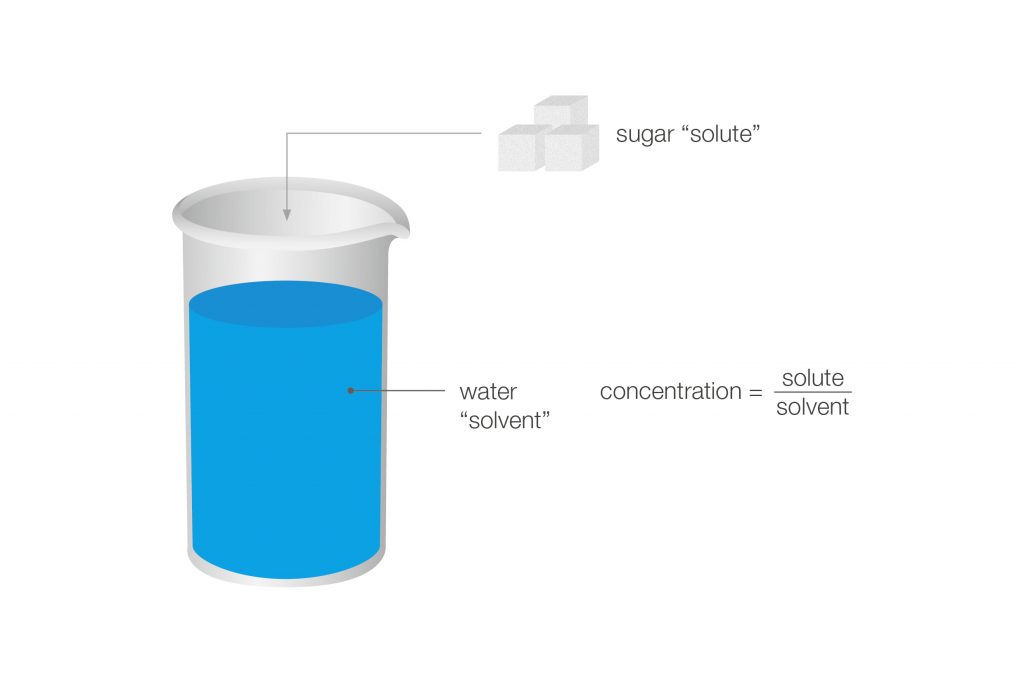
Figure 1: By adding a substance to a solvent, the density of the mixture will be different from the density of the pure solvent. Density can therefore be used to determine the concentration of a substance (“solute”) in a solution (e.g. water).
Binary and quasi-Binary Mixtures
Two-component mixtures are called binary mixtures. The density of the mixture is a function of its composition. Thus, the density value of a binary mixture can be used to calculate its composition with the aid of concentration tables.
Typical two-component mixtures are e.g. alcohol-water solutions, sugar-water solutions, salt-water solutions, and acids or bases dissolved in water.
Concentration determination is also possible with so-called quasi-binary mixtures. These are mixtures containing two major components and some additional ingredients which are present in very small concentrations compared to the two main components. Due to their small impact on the bulk density these additional ingredients can be ignored.
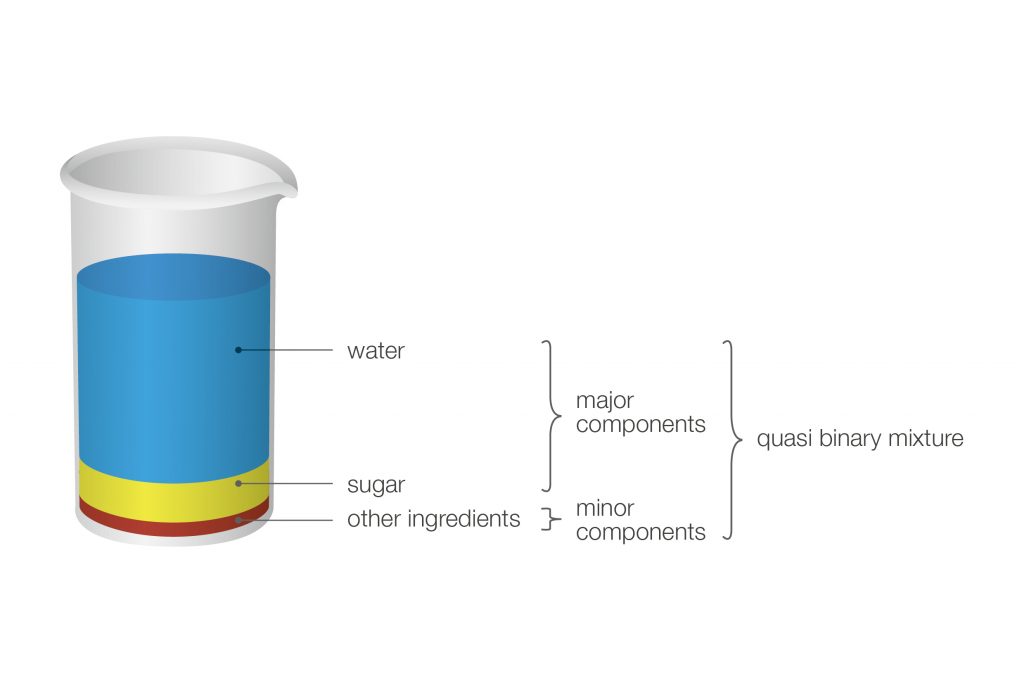
Figure 2: The major components of regular soft drinks are water and sugar (saccharose), all other ingredients, such as flavors or food coloring, are present in an insignificant amount. Therefore, soft drinks are considered sugar-water solutions and density measurement is used to determine the sugar content.
Conversion of Density to Concentration
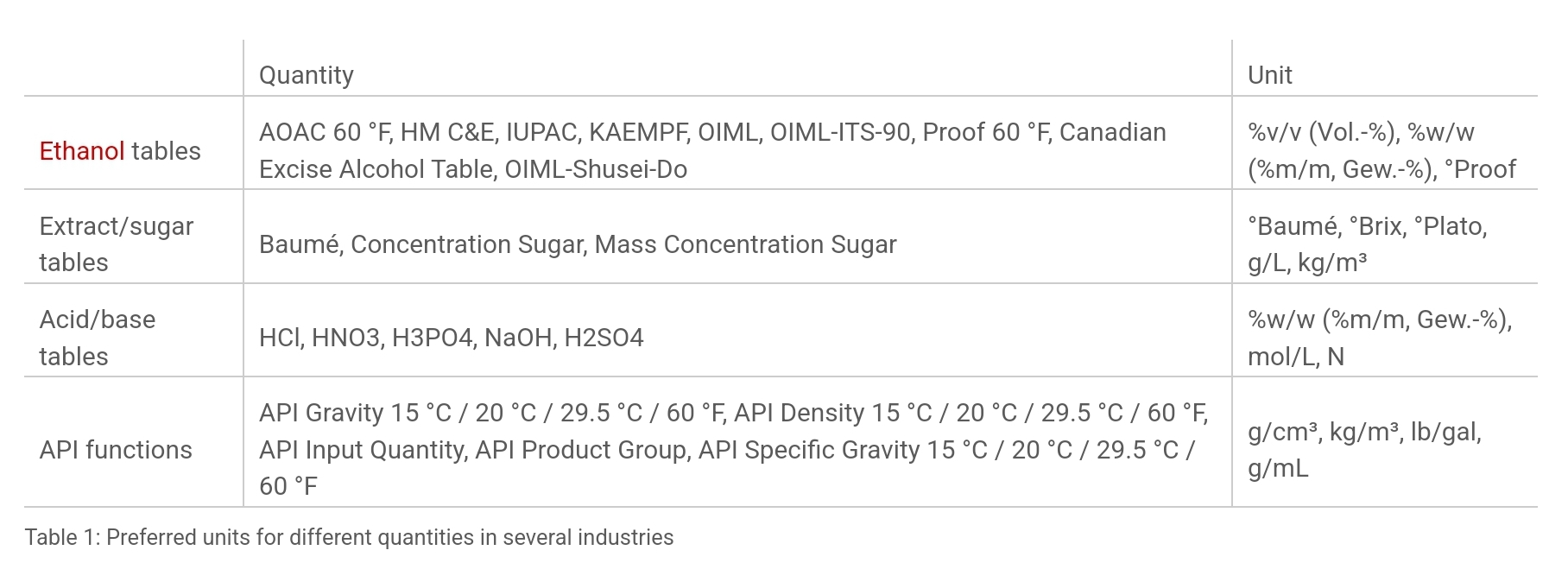
The density of a mixture can be converted into a concentration unit using a suitable concentration table or, more generally, a conversion formula. Nearly all binary solutions can be characterized by using density-related functions and concentration tables. These tables may either come from literature or individual experimental data.
The concentration of the solutions or mixtures is often expressed in terms of the solution’s percentage composition on weight or volume basis.
Density Measurement With Oscillating U-tube
The density is defined as the property of a body, given by the ratio of its mass to its volume (kg/m3). The fluid density is a major key in the control of most industrial processes, because it allows not only, a better management of the process but also the accurate determination of the quantity and quality of the product. The measurement of the density is used in drinks industry, to control the alcohol content in binary mixtures or the sugar content in soft drinks and fruit juices; in the pharmaceutical industry, to determine the specific gravity of medicinal preparations; in the oil industry to determine the API gravity and for the quality control of fuels and additives; in the chemical and nuclear, to determine the concentration of acids, bases and other solutions and to determine radioactive substances concentration; in the food industry, cosmetics, etc.
This magnitude can be determined using several measuring instruments, such as pycnometers, hydrometers and oscillation-type density meters, and also using the method of hydrostatic weighing. Recently the oscillationtype density meters have shown great versatility, and are being used in various branches of industry.
In 1967, the company Anton Paar GmbH presented the first digital density meter for liquids and gases. It was the first instrument to use the principle of the U shaped vibrating tube, of Stabinger Hans and Hans Leopold for the determination of the density. These first oscillation-type density meters were subject to errors induced by the viscosity in most of the samples. These errors were usually higher than 0,7 kg/m3 in instruments with a resolution of 0,1 kg/m3. In 1998, Hans Stabinger developed a density meter (Paar DMA 5000) with a new measurement cell which applied the viscosity correction of the sample, thereby avoiding the systematic errors of the others instruments.
The working principle of an oscillation-type density meter is based on the law of harmonic oscillation, in which a U tube is completely filled with the sample to be analyzed, and subjected to an electromagnetic force. The measurement of the frequency and duration of vibration of the tube filled with the sample, allows the determination of the density value of the sample. This measuring principle is based on the Mass-Spring Model.
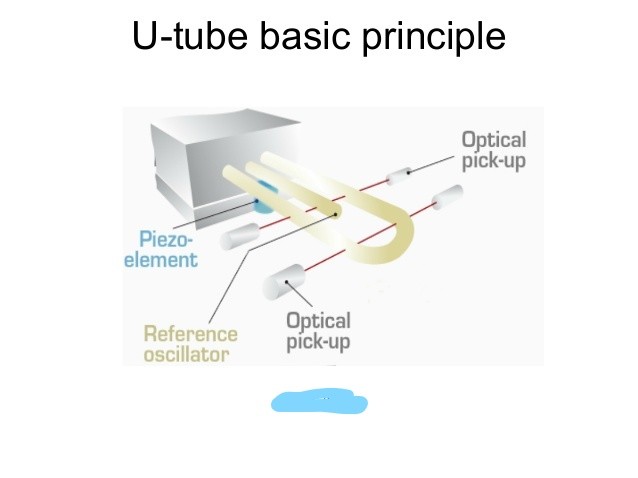
Modern high-precision density meters additionally provide a viscosity correction and a reference oscillator to enable accurate results over a large range of densities, temperatures, and viscosities. The U-tube method is used to measure the true density ρ of fluids.

Example: Concentration Determination of Sulfuric Acid and Oleum Using Density and Sound Velocity
Sulfuric acid is amongst the world’s large volume chemicals and found in various industries as a central substance. The biggest industry in need for sulfuric acid is the fertilizer production. Other uses include the metal industry, the petroleum industry and the chemical industry. Anton Paar offers density and density and sound velocity meters for easy and precise concentration determination.
A standard method for concentration determination of sulfuric acid and oleum is titrating sulfuric acid or oleum with a base, commonly sodium hydroxide (NaOH). The titration of acid and oleum requires not only the dilution of the samples with distilled water, but is also dependent on the operator skills.
The Anton Paar DSA 5000 M density and sound velocity meter uses an efficient method for concentration determination of sulfuric acid and oleum by combining two physical properties, density and sound velocity. A specific measuring method "Sulfuric Acid & Oleum" was defined for DSA 5000 M, allowing the fast and accurate determination of sulfuric acid and oleum samples of any concentration.
In order to compare DSA 5000 M results with values obtained by titration different concentrations of sulfuric acid and oleum were measured. The measurement results show a very good agreement between titration and DSA 5000 M. Nevertheless, it should be noted that the standard deviation of titration results is constantly higher than of DSA 5000 M measurements.
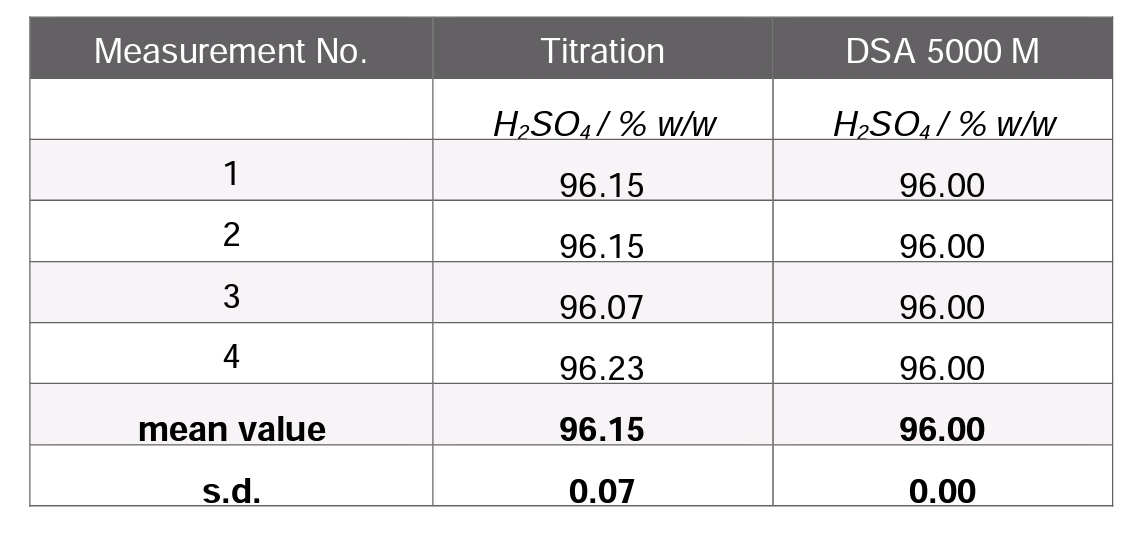
Table 2: Comparison of titration and DSA 5000 M results, incl. mean value and standard deviation (s.d.); sample: 96 % w/w H2SO4
References:
- https://www.aatbio.com/resources/faq-frequently-asked-questions/what-s-the-relationship-between-concentration-of-solution-and-its-density
- https://wiki.anton-paar.com/en/concentration-determination-means-density-measurement/
- MEASUREMENT OF DENSITY USING OSCILLATION-TYPE DENSITY METERS CALIBRATION, TRACEABILITY AND UNCERTAINTIES A. Furtado, E. Batista, I. Spohr, E. Filipe Instituto Português da Qualidade (IPQ) Rua António Gião, 2 – Caparica – Portugal
- https://extranet.anton-paar.com/extranet/wwwportal.nsf/api.xsp/download?app=docs&id=B26FB909985AB4DBC125832E00476A7C
Prime Scientific
Prime Scientific provides equipment, instruments and other related products and materials. We aim to be a one stop shop for all our customers laboratory needs. Since 2001, Prime has strived to provide the best services and solutions to the scientific community in the region.